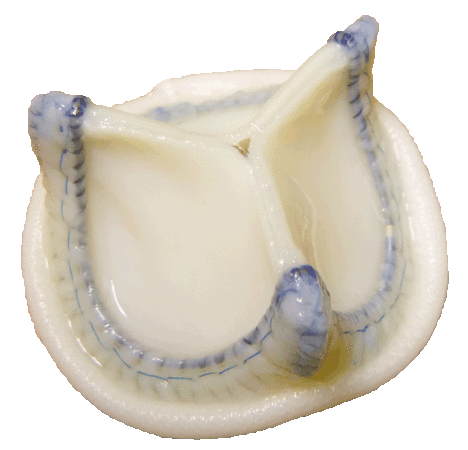
Biological Heart Valve Prosthesis
|
|
|
|
Offers ease of implantation and optimal hemodynamic performance.
It has been used in clinical practice since 2011.
 |
Video |
ЮниЛайн Аортальный - каркасный клапан со створками из бычьего перикарда предназначен для супрааннулярной имплантации в аортальную позицию.
Конструкция и материалы
В конструкции клапана использован композитный каркас, состоящий из полипропиленовой формы и суперэластичной проволоки с памятью формы. Обшивка каркаса клапана биологической тканью позволяет проводить специальную обработку всей поверхности.
Для удобства имплантации манжета клапана отчасти определяет контур, который соответствует форме нативного фиброзного кольца аортального клапана.
Створки клапана изготавливаются из перикарда крупного рогатого скота. Консервация, стерилизация и химическая сшивка биологического материала производится с использованием диглицидилового эфира этиленгликоля. Биоматериал фиксированный по технологии применяемой компанией НеоКор демонстрирует высокую устойчивость от накопления солей кальция и долговечность по отдаленным клиническим результатам.
Консервированный биоматериал подвергается дополнительным обработкам, придающим клапану собственную антибактериальную активность или высокую резистентность к кальцификации.
Прецизионное изготовление створок
- Высокоточный раскрой створок с использованием лазерной установки, позволяющей
полностью избежать разволокнения коллагеновых волокон по краю среза;
- Компьютерная технология изготовления створок с детекцией толщины перикарда перед раскроем, обеспечивает стабильное качество створчатого аппарата каждого протеза. Максимальная однородность створчатого аппарата по толщине способствует равномерному распределению нагрузки по всей поверхности створки;
Выходной контроль при производстве
- Каждый клапан ЮниЛайн проходит испытания на гидродинамическом стенде на соответствие требованиям установленным ГОСТ 31618.1-2012 Протезы клапанов сердца. Общие технические требования и методы испытаний. Гидродинамические показатели EOA, транспротезный градиент и обратный переток фиксируются для предоставления этой информации хирургу в идентификационной карте, прилагаемой к каждому клапану.
Для удобства хирурга протез поставляется в комплекте с ручкой-держателем для имплантации.

Габаритные размеры клапана

|
Посадочный диаметр (размер), мм |
Наружный диаметр, мм |
Аортальный выступ, мм |
Высота, мм |
|
21±1 |
23±1 |
10,5±1,5 |
14,5±1,5 |
|
23±1 |
22±1 |
11,5±1,5 |
15,5±1,5 |
|
25±1 |
27±1 |
12,5±1,5 |
16,5±1,5 |
Функциональные характеристики биопротезов ЮниЛайн, полученные при стендовых испытаниях (данные производителя)
|
Показатель |
Посадочный диаметр (размер), мм |
||
|
21 |
23 |
25 |
|
|
Пропускная способность (МО), см3/цикл |
90+3 |
96+6 |
108+10 |
|
Обратный переток, см3/цикл |
3,027+1,19 |
2,98+0,63 |
3,73+0,89 |
Гемодинамические характеристики аортальных биопротезов ЮниЛайн в зависимости от диаметра протеза
|
Показатель |
Посадочный диаметр (размер), мм |
||
|
21 |
23 |
25 |
|
|
ЭПО (см2), min-max |
1,79+0,2 1,77-1,81 |
1,97+0,09 1,88-2,06 |
2,07±0,10 1,97-2,17 |
|
∆P макс., мм рт.ст., min-max |
18,1+5,3 8-31 |
17,9+5,0 9-33 |
18,4±6,2 6-32 |
|
∆Р сред., мм рт.ст., min-max |
13,8+4,1 6-27 |
10,0+3,6 4,6-30 |
8,0±3,1 3,8-13 |
Примечание: ЭПО - эффективная площадь открытия , ∆P макс.- максимальный градиент на аортальном клапане, ∆P сред. - средний градиент на аортальном клапане, в верхней строке указаны средние значения, в нижней – минимальное и максимальное значения.
- Антикальциевая. Выполняется по умолчанию если в заказе не указан тип обработки.
(Статья: Применение аминодифосфоната для профилактики кальцификации эпоксиобработанных биопротезов)
- Антитромботическая. Выполняется если в заказе указан соответствующий тип обработки.
- Антибактериальная. Выполняется если в заказе указан соответствующий тип обработки.
(Статья: "Новое поколение биопротезов клапанов сердца, обладающих повышенной тромборезистентностью и антибактериальной активностью")
Все биопротезы для кардиохирургии компании НеоКор по умолчанию производятся с антикальциевой обработкой для пациентов с прогнозируемым повышенным риском кальцинирования биопротеза (коррекция врожденных пороков у детей и приобретенных пороков до 50 лет у взрослых пациентов) Использование аминодифосфонатов способствует статистически значимому снижению кальций связывающей активности, не оказывая отрицательного влияния на структуру и физико-механические свойства биоматериала.
По заказу хирургов биопротезы обрабатываются гепарином для снижения риска тромбообразования. При проведении операции на фоне инфекционного эндокардита целесообразно заказать и использовать биопротезы компании НеоКор с антибактериальной обработкой.
Сообщите о необходимой Вам дополнительной обработке биопротеза при заказе изделия.
Для заказа направьте заявку по адресу neocor@neocor.ru или воспользуйтесь формой обратной связи на сайте.
Вы можете получить консультацию по медицинским изделиям компании НеоКор по контактным телефонам коммерческого отдела с 9.00 до 17.00 (разница с Москвой +4 часа) в рабочие дни.
Варианты исполнения клапана:
|
Посадочный диаметр, мм (пред. октл. ±1,0) |
Номер в каталоге |
|
|
21 |
ULA21b(s) |
|
|
23 |
ULA23b(s) |
|
|
25 |
ULA25b(s) |
|
|
При заказе дополнительно укажите: |
||
|
тип пришивной манжеты |
b - биологическая s - синтетическая |
|
|
тип обработки |
- антикальциевая (по умолчанию); - антитромботическая; - антибактериальная. |
|
В комплект поставки входит:
|
Наименование комплектующего |
Количество, шт |
|
Клапан ЮниЛайн |
1 |
|
Держатель имплантационный (Ручка и держатель) |
1 |
|
Индивидуальный контейнер с раствором для хранения |
1 |
|
Инструкция по применению |
1 |
|
Идентификационная карта |
1 |